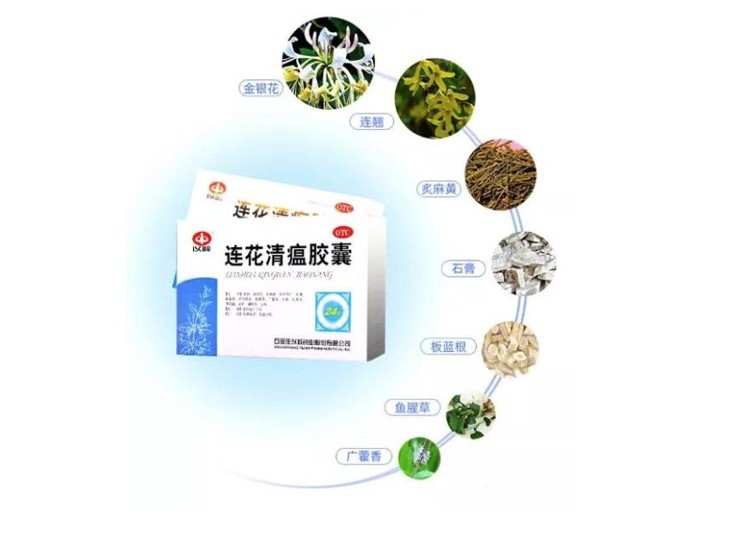
自新冠病毒疫情发生以来,连花清温制剂在治疗新冠病毒肺炎受到国际各界人士的高度评价和瞩目。连花清瘟方由中医经典方剂麻杏石甘汤与银翘散化裁,具有清瘟解毒,宣肺泄热的功效。药物组成包括连翘、金银花、炙麻黄、炒苦杏仁、石膏、板蓝根、绵马贯众、鱼腥草、广藿香、大黄、红景天、薄荷脑、甘草。连花清瘟制剂中含有环烯醚萜、木脂素、蒽醌、酚酸、黄铜等有效成分。
连花清温中连翘的主要成分金丝桃苷、芦丁和连珠苷E是抑制SARS-CoV-2病毒主要蛋白酶的重要抑制剂。连花清瘟可通过抑制IKK/IB/NF-kB信号通路,抑制炎性细胞浸润,改善肺泡上皮细胞和肺血管内皮细胞连接蛋白的表达,减轻脂多糖(LPS)导致的肺损伤。此外,动物实验证实连花清瘟对机体感染病毒具有免疫调节作用,增加流感病毒甲型鼠肺适应株(FM1)感染后CD4+和CD4+/CD8+ T细胞表达水平,提高小鼠流感病毒后肺组织中IFN-水平。
连花清瘟制剂在2020年2月被证实列入中国国家卫健委《新型冠状病毒肺炎诊疗方案(试行第6版)》,可用于轻型、普通型新冠肺炎引起的发热、咳嗽、乏力。一项随机对照试验显示连花清瘟胶囊能明显抑制COVID-19主要症状(发热、咳嗽),并且缩短病程,安全性良好。另一项随机对照试验显示连花清瘟胶囊能明显控制甲型流行性感冒的主要症状(发热、咳嗽、咽痛和乏力),并且能减轻疾病的严重程度,未报道不良事件发生。另一项随机对照试的结果显示连花清瘟颗粒能明显减轻肺炎支原体肺炎患儿的临床症状和体征,并且降低患儿的肺部炎症程度和炎性渗出,从中改善肺通气功能。
一项系统性评价显示连花清瘟制剂的一般安全性良好,不良事件的发生较西医相对少。连花清瘟制剂最常见的不良反应多涉及消化系统,包括恶心、呕吐、腹泻。皮肤和实验室检查方面的不良反应在临床中少见。联用连花清瘟制剂的不良反应高于单用连花清瘟制剂,因此可推测是联用的西药引起的不良反应。然未来需要展开更多大样本、多中心临床随机对照试验研究,以更科学、更客观地评价此药的功效。
References
- 成蕾, 刘洪, 王婷婷, 等. 连花清瘟制剂化学成分、药理作用、临床应用的研究进展.中成药. 2021,43(12):3409-3416.
- 尹玉洁, 常丽萍. 中药连花清瘟胶囊/颗粒在呼吸系统疾病中的药理研究及临床应用进展. 中国临床药理学与治疗学. 2021,10:1174-1180.
- Hu K, Guan WJ, Bi Y, et.al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine: international journal of phytotherapy and phytopharmacology.2021,85,153242.
- 牛明, 王睿林, 王仲霞, 等. 基于临床经验和分子对接技术的抗新型冠状病毒中医组方快速筛选模式及应用. 中国中药杂志. 2020,45(06):1213-1218.
- Ho TY, Wu SL, Chen JC,et.al. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007,74(2):92-101.
- 董勇, 张希洲, 占景琼, 等. 大黄萃取液对心肺复苏后兔肺TNF-α及IL-8表达的影响. 临床急诊杂志. 2017,18(05):366-368.
- Duan ZP, Jia ZH, Zhang J, et.al. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chin Med J (Engl). 2011,124(18):2925-33.
- 陈团营, 朱珊, 边红恩, 等. 连花清瘟颗粒对小儿肺炎支原体肺炎肺功能指标、血清炎症因子水平影响及疗效分析. 中华中医药学刊. 2018,36(11):2713-2715.
- 孙伟, 杨晓宇, 周璇, 等. 连花清瘟制剂临床用药安全的系统性评价. 世界中医药.2021,16(18):2727-2734.
Click to download PowerPoint